Cagent Vascular Completes Enrollment of PRELUDE Study Using Serranator Device
Cagent Vascular, a developer of next generation technology for vessel dilatation in cardiovascular disease interventions, including the FDA 510(k) cleared Serranator® Alto PTA Serration Balloon Catheter, announces the completion of enrollment in the First-in-Human PRELUDE Study.
The purpose of this prospective, single-arm, multicenter feasibility study is to show the safety and efficacy of the Serranator® Alto device used in the Superficial Femoral Artery (SFA) and/or Popliteal Artery.
The PRELUDE study is led by Principal Investigator Dr. Andrew Holden (Auckland, New Zealand). Other investigators participating in this study are Drs. Marianne Brodmann (Graz, Austria), Marek Krzanowski (Kraków, Poland) and Przemyslaw Nowakowski (Chrzanów, Poland). The centers enrolled 25 subjects and will participate in a 30-day and 6-month follow-up.
“The PRELUDE Trial evaluating the first in human experience of the Serranator® device has rapidly completed enrollment. Excellent acute results were achieved with 100% device success and a very low bail out stent rate despite a significant number of lesions containing chronic total occlusions and severe calcification. Interestingly, IVUS and OCT confirmed serrations were visible in all imaged patients. We look forward to following these patients as the trial progress,” said Dr. Holden.
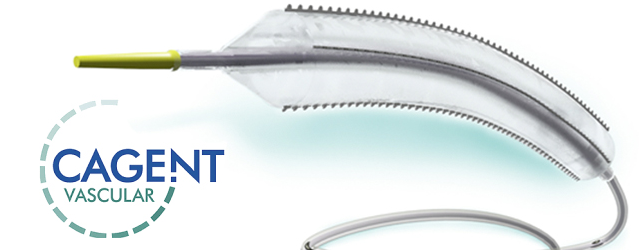
The Serranator® Alto device has four external metal serrated strips embedded on a semi-compliant balloon and is designed to create multiple longitudinal lines of interrupted micro-serrations to aid arterial expansion. The device received FDA 510(k) clearance earlier this year. The Serranator® Alto PTA Serration Balloon Catheter is intended for dilatation of lesions in the iliac, femoral, ilio-femoral, and popliteal arteries, and for the treatment of obstructive lesions of native or synthetic arteriovenous dialysis fistulae. (Not for use in the coronary or neuro-vasculature.)
Read the full article here.